
Additive Manufacturing
Additive manufacturing (AM) is a common technique used to assemble three dimensional (3D) structures for education or functional use. A group of processed materials is printed with a layered production technique to reach a targeted 3D model. Optimum success is attained in bone and cardiac tissue engineering using AM techniques [1,2].
Selective Laser Melting
SLM method belongs to PBF technology, whereby functional parts can be manufactured in a powder bed appliying the energy of a laser beam. Energy required for melting is provided to selected areas with the help of a high-intensity and focused laser source depending on desired layer geometry on the previously prepared powder bed. The powder particles in these areas melt and then solidify with very fast cooling, ensuring favorable bond to the substrate or the base plate used. In this way, high mechanical properties are obtained after the process. The entire production is carried out in a build chamber loaded with a protective gas (Nitrogen or Argon) to prevent oxidation at high temperatures [3–5].
There are over 300 process parameters defined in the field. In addition to these parameters, which affect the part fabrication performance, their interactions can have crucial effects on the outputs. For example, some SLM parameters combined in volumetric (E) or linear energy density (LED) equations can be seen as Eq 1 and Eq 2. The main parameters, which are laser power, scanning speed, laser focal diameter and layer thickness, can be combined to calculate the volumetric energy density. As indicated Equation 1, where E (J/mm3) symbolizes the volumetric energy density, P symbolizes laser power (W), v describes as scanning speed (mm/s), t is the layer thickness (µm) and s is the hatch scan spacing (mm) [6]. E determines the operating temperature, while the LED (J/mm, P/υ) determines the density of the part [7].
In optimization of SLM process parameters, many performance criteria are taken into account, not only high density but also others such as material composition, residual stresses, productivity, surface quality, mechanical properties and geometric accuracy. Besides, the raw material characteristics such as powder particle size distribution, chemical composition, internal porosity, particle morphology, etc. and minor differences in the machine components, e.g., powder laying method or gas circulation dynamics, lead to the variations of process parameters. Numerous studies on different materials in this field can be seen in the literature for process development for different purposes [8–14].
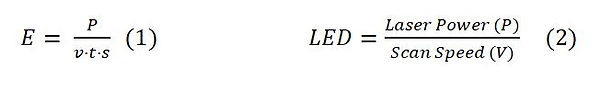
Titanium Mini Plates
Bone fracture repair and regeneration requires anatomical reduction and immobilisation by fixation (osteosynthesis). This will allow the reconstruction of blood circulation (neo-vascularisation) and recovery of the function [15]. Maxillofacial and hand / finger fractures need ultimate care as fixation can be limited by specific anatomy, proximity to nerves, vessels and tendons. Personal variations are frequent and soft tissue coverage is usually limited [16]. Commercial mini-plates of Ti or Ti6Al4V ensure stability in three dimensions in such fractures however the plates may not fit perfectly to the bone because commercial or additive manufactured cp-Ti mini plates have higher ductility properties than Ti6Al4V. This may cause decrease in mechanical strength and tissue compatibility [7]. Gilardino, Chen and Bartlett [17] determined inappropriate shape and tissue compatibility of plates as the main cause of a second surgery for removal. Another cause of removal of the osteosynthesis material is the disproportionate elastic (Young) modulus of the mini-plates. Elastic modulus of titanium (110 GPa) is closer to cortical bone compared to stainless steel (200 GPa) and cobalt-chromium (230 GPa) [18]. The application of cp-Ti and Ti6Al4V mini-plates and screws in maxillofacial and hand surgery has become worldwide accepted due to the high strength, corrosion resistance, lightness and osseointegration properties of the material [19, 20].
Triply Periodic Minimal Surfaces-Gyroid
Triply periodic minimal surfaces (TPMS) have been the mathematical models that have been focused on due to their unique biological and mechanical behavior. TPMS describes an infinite structure and three independent directions with an average zero curvature of the surface (Concave and convex curves are symmetrical at all points). Gyroid, which is one of them, will be the pore structure to be applied in the project due to its low density, high tensile strength. In nature, gyroid lattices can be found in nanoporous membranes, solar cells, structural coloring in butterfly wing scale, and similarity to trabecular bone structure. In addition, it has been demonstrated that the gyroid has permeability higher than 10 times due to the lack of size-limiting pore interconnections [21]. Gyroid functions Eq.3 and Eq.4 were taken from Yuan et al. [22] article. Eq 3. is the implicit function that is the expression of gyroid architecture. x, y, and z are in the cartesian coordinates. Eq 4. L is the size of a unit cell's edge length. a is offset value for determining volume fractions of unit cells.

Coating
If the cellular recognition and excellent chemical bonds with the hosted tissue are absent from surface of the implant, attachment of the implant will be inadequate and micro-motions will occur at the bone-implant interface. This situation will lead to a failure of the implant system. Along with the wear of the implant material, corrosion provokes the release of metal ions or particles and causes infection and immune response. These limitations are addressed by the surface coating method that protects the implant material and at the same time increases their surface biocompatibility [23, 24].
The most important requirement of any implant (metallic, polymeric, ceramic) is to prevent infection and bone resorption, as well as to increase osteointegration and biological stabilization. The deposition of nanocoatings and nanocomposite coatings on these implants is to increase biological activity, corrosion resistance, and protect the body from adverse reactions due to metal ion release. These coatings also allow the surface properties of the implant to be altered to achieve improvements in clinical reliability, longevity, and performance [25].
Calcium Phosphate
Calcium phosphate has been used as a porous coating on metal implants in orthopedic applications. The development of good interfacial strength between the bioceramic coating and bone tissue is the effect of biochemical interference of released calcium and phosphate ions [25]. Calcium phosphates are classified according to their solubilities such as when attached to surrounding tissues together with their ability to be degraded and replaced by advancing bone growth. Surface ions of calcium phosphate are replaced with the aqueous solution when in contact with body fluid. Various ions and molecules (protein and collagen), on the other side, can be adsorbed onto the surface of calcium phosphate bioceramics [26].
Silk Fibroin
Silk fibroin is a natural protein extracted from Bombyx mori, which have been actively used in the field of tissue engineering and regenerative medicine recently, and demonstrated its biocompatibility, biodegradability [27].
Dip Coating
One of the desired techniques of the sol-gel coating method is the dip coating which has some advantages over other methods such as minimum thickness, low operating temperature and low volume shrinkage [28]. It has also been found to be suitable for coating complex shapes. This process provides high thickness homogeneity up to 1000 nm, suitable for the production of multilayer [29].
References
[1] F. Trevisan, F. Calignano, A. Aversa, G. Marchese, M. Lombardi, S. Biamino, D. Ugues, D. Manfredi, Additive manufacturing of titanium alloys in the biomedical field: processes, properties and applications, J. Appl. Biomater. Funct. Mater. 16 (2018) 57–67. https://doi.org/10.5301/jabfm.5000371.
[2] G. Mirone, R. Barbagallo, F. Giudice, S. Di Bella, Analysis and modelling of tensile and torsional behaviour at different strain rates of Ti6Al4V alloy additive manufactured by electron beam melting (EBM), Mater. Sci. Eng. A. 793 (2020) 139916. https://doi.org/10.1016/j.msea.2020.139916.
[3] L.C. Zhang, Y. Liu, S. Li, Y. Hao, Additive Manufacturing of Titanium Alloys by Electron Beam Melting: A Review, Adv. Eng. Mater. 20 (2018). https://doi.org/10.1002/adem.201700842.
[4] S. Liu, Y.C. Shin, Additive manufacturing of Ti6Al4V alloy: A review, Mater. Des. 164 (2019) 107552. https://doi.org/10.1016/j.matdes.2018.107552.
[5] C. Taltavull, B. Torres, A.J. López, P. Rodrigo, E. Otero, J. Rams, Selective laser surface melting of a magnesium-aluminium alloy, Mater. Lett. 85 (2012) 98–101. https://doi.org/10.1016/j.matlet.2012.07.004.
[6] B. Vandenbroucke, J.P. Kruth, Selective laser melting of biocompatible metals for rapid manufacturing of medical parts, Rapid Prototyp. J. 13 (2007) 196–203. https://doi.org/10.1108/13552540710776142.
[7] A. Ataee, Y. Li, M. Brandt, C. Wen, Ultrahigh-strength titanium gyroid scaffolds manufactured by selective laser melting (SLM) for bone implant applications, Acta Mater. 158 (2018) 354–368. https://doi.org/10.1016/j.actamat.2018.08.005.
[8] E. Yasa, J. Kruth, Application of laser re-melting on selective laser melting parts, Adv. Prod. Eng. Manag. 6 (2011) 259–270.
[9] K. Kempen, E. Yasa, L. Thijs, J.P. Kruth, J. Van Humbeeck, Microstructure and mechanical properties of selective laser melted 18Ni-300 steel, in: Phys. Procedia, Elsevier B.V., 2011: pp. 255–263. https://doi.org/10.1016/j.phpro.2011.03.033.
[10] K.M.C. Viramgama M.D., Viramgama M.D., Karia M.C. Study and investigation of influence of process parameters for selective laser melting - a Review [PDF] - Все для студента, (n.d.). https://www.twirpx.com/file/2142723/.
[11] W. Huang, J. Yang, H. Yang, G. Jing, Z. Wang, X. Zeng, Heat treatment of Inconel 718 produced by selective laser melting: Microstructure and mechanical properties, Mater. Sci. Eng. A. 750 (2019) 98–107. https://doi.org/10.1016/j.msea.2019.02.046.
[12] S.D. Jadhav, S. Dadbakhsh, L. Goossens, J.P. Kruth, J. Van Humbeeck, K. Vanmeensel, Influence of selective laser melting process parameters on texture evolution in pure copper, J. Mater. Process. Technol. 270 (2019) 47–58. https://doi.org/10.1016/j.jmatprotec.2019.02.022.
[13] Y. Li, J. Zhou, P. Pavanram, M.A. Leeflang, L.I. Fockaert, B. Pouran, N. Tümer, K.U. Schröder, J.M.C. Mol, H. Weinans, H. Jahr, A.A. Zadpoor, Additively manufactured biodegradable porous magnesium, Acta Biomater. 67 (2018) 378–392. https://doi.org/10.1016/j.actbio.2017.12.008.
[14] X. Duan, X. Chen, K. Zhu, T. Long, S. Huang, F.Y.H. Jerry, The Thermo-Mechanical Coupling Effect in Selective Laser Melting of Aluminum Alloy Powder, Materials (Basel). 14 (2021) 1673. https://doi.org/10.3390/ma14071673.
[15] Y. Okazaki, E. Gotoh, J. Mori, Strength–Durability Correlation of Osteosynthesis Devices Made by 3D Layer Manufacturing, Materials (Basel). 12 (2019) 436. https://doi.org/10.3390/ma12030436.
[16] C. Martínez-Villalobos S. Castillo, Maxillofacial osteosynthesis with titanium. Revista Española de Cirugía Oral y Maxilofacial, 26(2004), 351-368.
[17] M.S. Gilardino, E. Chen, S.P. Bartlett, Choice of Internal Rigid Fixation Materials in the Treatment of Facial Fractures, Craniomaxillofac. Trauma Reconstr. 2 (2009) 49–60. https://doi.org/10.1055/s-0029-1202591.
[18] N. Balaban, Titanyum ve alaşımlarının biyouyumluluklarının incelenmesi, Fen Bilimleri Enstitüsü, 2007. https://polen.itu.edu.tr/handle/11527/9296.
[19] G. Mittal, R.R. Dubbudu, K.M. Cariappa, Three Dimensional Titanium Mini Plates in Oral & Maxillofacial Surgery: A Prospective Clinical Trial, J. Maxillofac. Oral Surg. 11 (2012) 152–159. https://doi.org/10.1007/s12663-011-0267-0.
[20] A. Chatterjee, B. Sapru, P. Awasthi, Effıcacy of ındıgenously manufactured tıtanıum bone plates and screws ın maxıllofacıal surgery, Med. J. Armed Forces India. 55 (1999) 287–290. https://doi.org/10.1016/s0377-1237(17)30349-0.
[21] D.J. Yoo, Leading Opinion Porous scaffold design using the distance field and triply periodic minimal surface models q, (2011). https://doi.org/10.1016/j.biomaterials.2011.07.019.
[22] L. Yuan, S. Ding, C. Wen, Additive manufacturing technology for porous metal implant applications and triple minimal surface structures: A review, Bioact. Mater. 4 (2019) 56–70. https://doi.org/10.1016/j.bioactmat.2018.12.003.
[23] A. Bartkowiak, K. Suchanek, E. Menaszek, B. Szaraniec, J. Lekki, M. Perzanowski, M. Marszałek, Biological effect of hydrothermally synthesized silica nanoparticles within crystalline hydroxyapatite coatings for titanium implants, Mater. Sci. Eng. C. 92 (2018) 88–95. https://doi.org/10.1016/j.msec.2018.06.043.
[24] H.T. Sirin, I. Vargel, T. Kutsal, P. Korkusuz, E. Piskin, Ti implants with nanostructured and HA-coated surfaces for improved osseointegration, Artif. Cells, Nanomedicine Biotechnol. 44 (2016) 1023–1030. https://doi.org/10.3109/21691401.2015.1008512.
[25] A.H. Choi, S. Cazalbou, B. Ben-Nissan, Nanobiomaterial Coatings in Dentistry, Front. Oral Biol. 17 (2015) 49–61. https://doi.org/10.1159/000381693.
[26] A.H. Choi, B. Ben-Nissan, Calcium phosphate nanocomposites for biomedical and dental applications: Recent developments, in: Handb. Compos. from Renew. Mater., wiley, 2017: pp. 423–450. https://doi.org/10.1002/9781119441632.ch163.
[27] S.H. Kim, Y.K. Yeon, J.M. Lee, J.R. Chao, Y.J. Lee, Y.B. Seo, M.T. Sultan, O.J. Lee, J.S. Lee, S. Il Yoon, I.S. Hong, G. Khang, S.J. Lee, J.J. Yoo, C.H. Park, Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing, Nat. Commun. 9 (2018) 1–14. https://doi.org/10.1038/s41467-018-03759-y.
[28] H. Najafi, A. Nemati, Z. Sadeghian, Crystallisation kinetics of hydroxyapatite thin films prepared by sol-gel process, Adv. Appl. Ceram. 109 (2010) 313–317. https://doi.org/10.1179/174367609X422144.
[29] A.H. Choi, B. Ben-Nissan, A. Bendavid, Thin films and nanocoating of Hydroxyapatite on titanium implants, LAP LAMBERT Academic Publishing, ISBN-10: 3330064250 (2017).